Hospitals are meant to heal, but some devices meant to save lives ended up doing serious harm. Over the decades, these medical tools were pulled from shelves and banned from use after being linked to deaths, permanent injuries, or widespread malfunctions that shook trust in healthcare safety protocols.
Dalkon Shield IUD

Approved in the early 1970s, the Dalkon Shield was a contraceptive device that ended up causing severe infections, sepsis, and infertility in thousands of women. Its multifilament string wicked bacteria straight into the uterus. After 18 deaths and over 300,000 lawsuits, it was banned in 1974 and remains a textbook case of medical negligence.
Therac-25 Radiation Machine
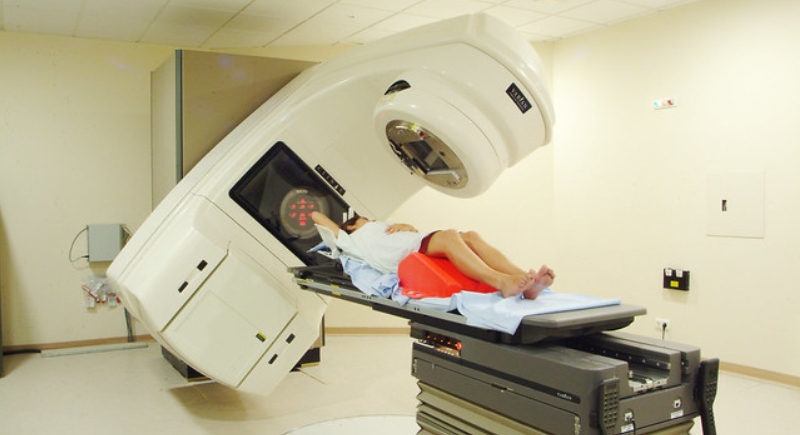
This computer-controlled radiation therapy machine tragically overdosed at least six patients between 1985 and 1987, killing three. A software bug allowed massive radiation levels to be administered. Operators had no idea the dosage was off until patients suffered burns and neurological damage. It was pulled after a damning FDA investigation.
Metal-on-Metal Hip Implants
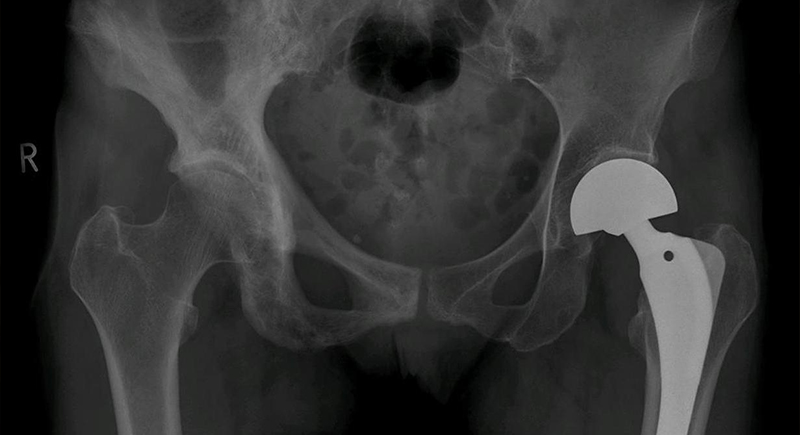
These implants were promoted in the 2000s as more durable than ceramic or plastic versions, but started shedding toxic cobalt and chromium particles into patients’ bloodstreams. This caused tissue death, inflammation, and even heart and nervous system issues. By 2013, most models were discontinued in the U.S., with thousands of lawsuits filed against major manufacturers like DePuy and Stryker.
Power Morcellators
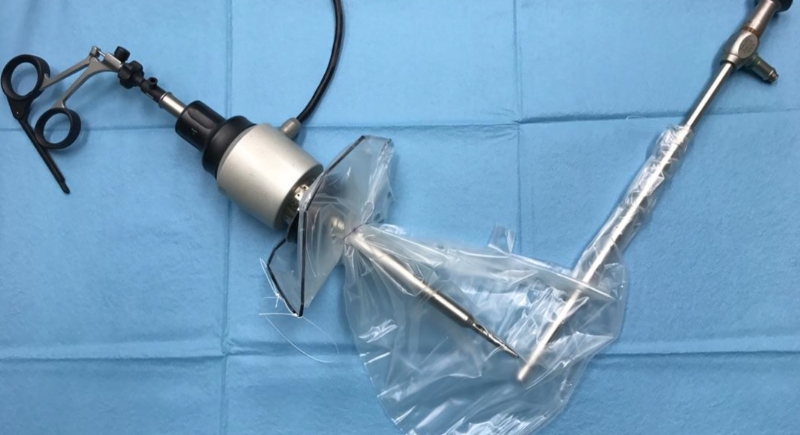
Power morcellators were used during minimally invasive hysterectomies to slice up uterine fibroids. Unfortunately, they unintentionally spread undiagnosed cancer cells throughout the abdominal cavity. The FDA issued a warning in 2014, and major hospital systems—including the Cleveland Clinic and Brigham and Women’s—soon stopped using them altogether. It’s estimated that 1 in 350 women had hidden cancers worsened by these devices.
Boston Scientific RotaWire Guidewire

The RotaWire was pulled from hospitals in 2021 after it was discovered to break inside arteries during heart procedures, which led to strokes and emergency surgeries. The FDA categorized it as a Class I recall—the most serious kind—after nearly 50 adverse events were reported, including deaths.
Silicone Breast Implants (Pre-2006)

These early implants often ruptured and leaked silicone into surrounding tissue and sometimes into the bloodstream. Though long debated, studies linked them to autoimmune diseases, chronic fatigue, and connective tissue disorders. The FDA banned them in 1992, only allowing reintroduction in 2006 with tighter safety testing.
Transvaginal Mesh
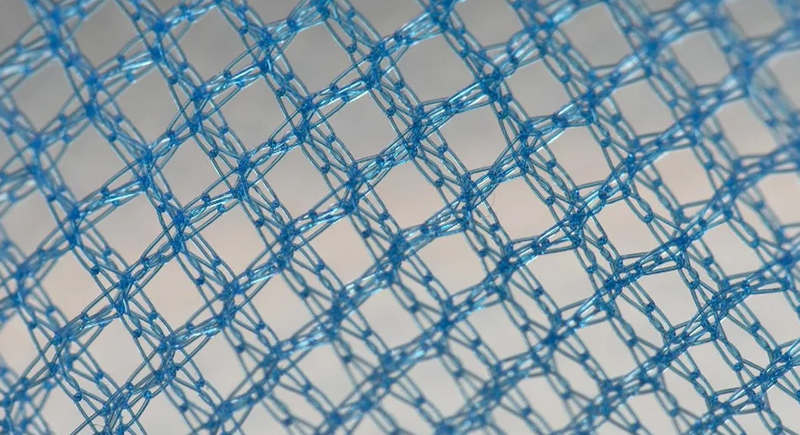
Marketed heavily in the early 2000s for pelvic organ prolapse and urinary incontinence, transvaginal mesh began causing pain, bleeding, and even organ perforation. Tens of thousands of women reported complications, and the FDA reclassified it as high-risk in 2016 before finally banning it entirely in 2019 for these conditions.
Radiation-Emitting Shoe-Fitting Fluoroscopes

In the 1940s and 1950s, these X-ray machines let kids see their toes wiggle inside new shoes. There was no lead shielding, no time limits—just pure radiation exposure. Studies later confirmed that store clerks and frequent users had higher cancer rates. The machines were banned in the U.S. by the 1970s.
Bair Hugger Warming System

This forced-air warming device, used in surgeries to keep patients warm, came under scrutiny after allegations that it increased infection risk in joint replacement patients. Though it’s still used in many places, several hospitals stopped using it following lawsuits suggesting the system disrupted sterile air around surgical sites.
Contaminated Heater-Cooler Units

These devices regulate body temperature during open-heart surgery. One model, the Stockert 3T, was linked to a rare bacterial infection—Mycobacterium chimaera—which could take years to show symptoms. The CDC estimated thousands might have been exposed. Hospitals had to replace or rigorously clean these units following multiple FDA warnings starting in 2015.
Infusion Pumps with Software Glitches

Between 2005 and 2009, the FDA received over 56,000 reports of problems with infusion pumps, including 710 deaths. Many stemmed from software bugs or confusing user interfaces. In response, the FDA cracked down on approvals and forced manufacturers to rethink design safety. Some models were pulled permanently from operating rooms.
Defective Heart Stents (Cypher Stent)

Early drug-eluting stents like the Cypher were praised for preventing restenosis, but they started showing signs of late stent thrombosis—sudden clots forming years later. A 2006 study published in the New England Journal of Medicine triggered serious concerns, and while not banned outright, their use sharply declined in hospitals.
Featherweight Oxygen Cylinders

Lightweight aluminum oxygen cylinders were found to explode when exposed to high heat or sparks, especially in ambulance settings. A major 1998 NIOSH report flagged the hazard after multiple EMT injuries and fatalities. Most hospitals and first responders switched to safer composite models after regulatory advisories were issued.
Rejuvenate Modular Hip System by Stryker
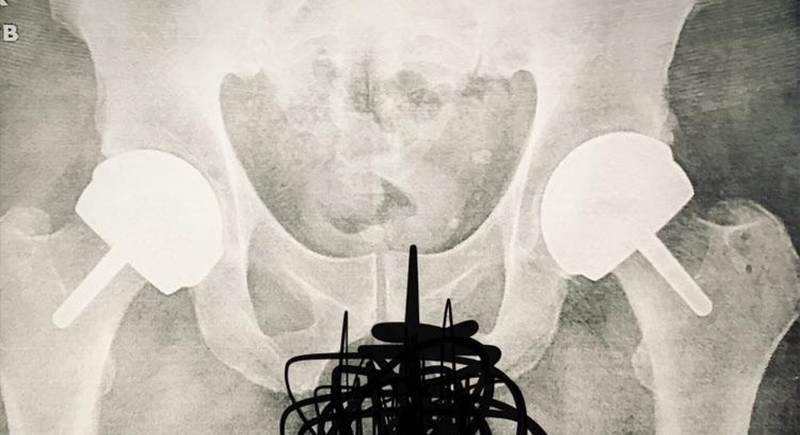
Released in 2009 and recalled in 2012, this hip implant was plagued by corrosion at the junctions, which caused metallosis—a condition where metal debris builds up in the tissue. Thousands of patients had to undergo painful revision surgeries, and the company paid over $1 billion in settlements.
Norplant Contraceptive Implants
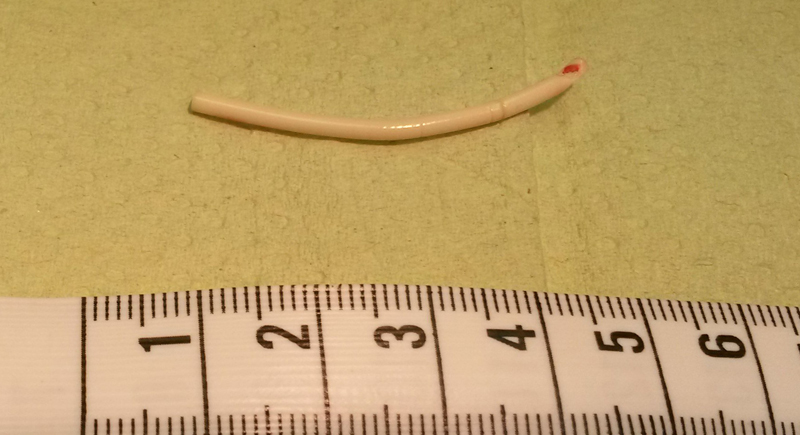
These implants were approved in 1990 and removed from the U.S. market by 2002. Norplant consisted of six small rods implanted in the arm for long-term birth control. Side effects included severe headaches, irregular bleeding, and depression. More than 36,000 women joined lawsuits and cited that doctors and manufacturers didn’t adequately warn them of potential complications.